Perché umidità capillare è una falsa definizione
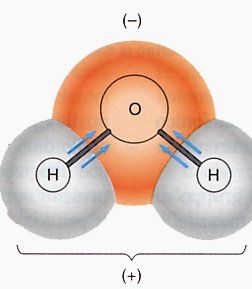 Il potere assorbente di un terreno o di un laterizio (che successivamente chiameremo supporto) è la proprietà che ha un suolo o un manufatto, di trattenere l’acqua e gli elementi soluti. umidità capillare
Il potere assorbente di un terreno o di un laterizio (che successivamente chiameremo supporto) è la proprietà che ha un suolo o un manufatto, di trattenere l’acqua e gli elementi soluti. umidità capillare
Solitamente l’acqua viene trattenuta grazie al concorso di due differenti fenomeni, l’imbibizione e la capillarità, il primo è di natura fisico-chimica ed il secondo è di natura prettamente fisica.
In entrambi i casi, la capacità di ritenuta è riconducibile a forze elettrostatiche che si instaurano fra le molecole d’acqua e la matrice solida del supporto (sia esso terreno o laterizio). La pressione che occorre esercitare per sottrarre l’acqua trattenuta dalla matrice solida è detta tensione matriciale ed assume generalmente valori negativi.umidità capillare
La ritenuta per capillarità è dovuta alla risultante delle forze che agiscono su ogni molecola d’acqua che occupa i pori del materiale; queste forze sono sempre riconducibili a quattro forze ben conosciute e distinte:
– forze gravitazionali che agiscono sulle molecole;
– forze di coesione interne fra le molecole d’acqua;
– forze di attrazione fra l’atmosfera del supporto e le molecole d’acqua;
– forze di adesione fra le molecole d’acqua e la superficie della matrice solida.
Sulle molecole disposte a breve distanza dalle particelle solide del supporto, la risultante è concorde alle forze di adesione, mentre sulle molecole relativamente distanti, si esercita una risultante concorde alla forza gravitazionale. In altre parole, laddove l’acqua risulta più lontana dalle particelle solide, questa è soggetta alla percolazione verso il basso perché attratta dalla forza di gravità, mentre dove la particella è vicina e stretta alle particelle solide è soggetta ad essere trattenuta perché attratta dalle forze di adesione. umidità capillare
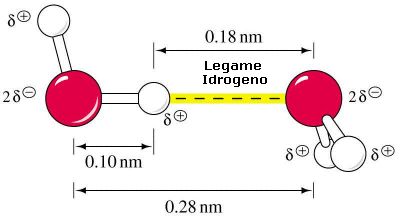 Ancora più semplicemente: in un sistema formato da due particelle solide disposte ad una distanza reciproca e lo spazio fra loro compreso, l’acqua che occupa questo spazio è soggetta a percolazione se la distanza supera un determinato valore, mentre è soggetta a ritenuta capillare se la stessa distanza è inferiore. Il valore di riferimento di questa distanza è di 8 µm: i pori con diametro inferiore a 8 µm (detti micropori) trattengono l’acqua per capillarità vincendo la forza di gravità, mentre quelli con diametro superiore (detti macropori) non riescono a trattenere l’acqua perché soggetta alla forza di gravità.
Ancora più semplicemente: in un sistema formato da due particelle solide disposte ad una distanza reciproca e lo spazio fra loro compreso, l’acqua che occupa questo spazio è soggetta a percolazione se la distanza supera un determinato valore, mentre è soggetta a ritenuta capillare se la stessa distanza è inferiore. Il valore di riferimento di questa distanza è di 8 µm: i pori con diametro inferiore a 8 µm (detti micropori) trattengono l’acqua per capillarità vincendo la forza di gravità, mentre quelli con diametro superiore (detti macropori) non riescono a trattenere l’acqua perché soggetta alla forza di gravità.
In condizioni ottimali di umidità e di aerazione quindi i micropori sono occupati dall’acqua ed i macropori dall’aria. umidità capillare
L’eventuale presenza di acqua nei macropori può essere:
– di carattere temporaneo in quanto si tratta di acqua libera e destinata a muoversi in verticale o lungo il profilo del supporto e comunque verso il basso. umidità capillare
– di carattere semi permanente in quanto si tratta di acqua imprigionata in accumuli salini tendenzialmente igroscopici che non ne consentono la discesa. umidità capillare
Per convenzione chiameremo:
– acqua capillare quella trattenuta nei micropori e si dispone staticamente formando menischi concavi in corrispondenza dell’interfaccia di separazione aria/acqua.
– acqua gravitazionale quella soggetta a movimento attraverso i macropori o che rimane imprigionata in essi a seguito di ritenzione idrica dovuta a componenti igroscopici o salini.
La ritenuta per imbibizione è dovuta alla proprietà delle particelle dotate di cariche elettriche superficiali di attirare l’acqua grazie alle forze elettrostatiche riconducibili al tipo ione-dipolo. L’attrazione elettrostatica è esercitata sia dagli ioni incorporati nel reticolo cristallino dei minerali, sia dai gruppi funzionali dissociati dei composti organici, sia dagli ioni adsorbiti sulla superficie dei colloidi. umidità capillare
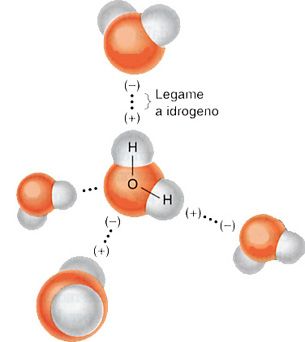 In virtù di queste forze, l’acqua tende a formare un velo il cui spessore dipende dall’intensità del campo elettrostatico generato dalle particelle solide. L’interazione fra acqua e particella solida è di tipo superficiale e dipende pertanto dalla densità elettrica della particella. Come tutti i fenomeni d’interfaccia superficiale, l’intensità di questa interazione aumenta all’aumentare del rapporto superficie/volume e, quindi, decresce all’aumentare delle dimensioni delle particelle. umidità capillare
In virtù di queste forze, l’acqua tende a formare un velo il cui spessore dipende dall’intensità del campo elettrostatico generato dalle particelle solide. L’interazione fra acqua e particella solida è di tipo superficiale e dipende pertanto dalla densità elettrica della particella. Come tutti i fenomeni d’interfaccia superficiale, l’intensità di questa interazione aumenta all’aumentare del rapporto superficie/volume e, quindi, decresce all’aumentare delle dimensioni delle particelle. umidità capillare
In pratica, le forze di attrazione sono:
– molto intense nel caso di particelle di dimensioni atomiche (ioni)
– relativamente intense nel caso di particelle di dimensioni colloidali (inferiore a 0,1 µm)
– molto deboli nel caso delle particelle di dimensioni macroscopiche (argilla di oltre 0,1 µm, limo, sabbia e scheletro).
La differente intensità delle forze d’attrazione si traduce nello sviluppo di strati d’idratazione di diverso spessore: gli ioni sono circondati da uno spesso strato di molecole d’acqua che ne determina la dispersione in soluzione; le particelle di dimensioni colloidali generano campi abbastanza intensi da legare gli ioni scambiabili e, attraverso la loro idratazione, uno strato di molecole d’acqua abbastanza spesso. Lo spessore di questo strato non ne permette la dispersione, perciò i colloidi del supporto flocculano restando allo stato di gel.
Solo nel caso di elevate quantità di sodio adsorbito si avrà un’idratazione tale da disperdere i colloidi. Le particelle più grandi, infine, generano un campo elettrico di modesta intensità e la quantità d’acqua che possono legare è modesta.
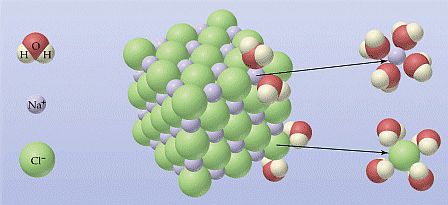 Sulla base di quanto detto, la capacità di un supporto di trattenere l’acqua (in condizione di assenza di contenuti igroscopici o salini) dipende in sostanza da due fattori: porosità totale e rapporto micropori/macropori e solo da essi dipende la capacità del supporto di legare l’acqua per capillarità.
Sulla base di quanto detto, la capacità di un supporto di trattenere l’acqua (in condizione di assenza di contenuti igroscopici o salini) dipende in sostanza da due fattori: porosità totale e rapporto micropori/macropori e solo da essi dipende la capacità del supporto di legare l’acqua per capillarità.
Queste due proprietà derivano dalla tessitura e dalla struttura:
– un supporto ricco di particelle grossolane avrà una modesta superficie di sviluppo, perciò una bassa porosità in gran parte rappresentata da macropori
– un supporto ricco di particelle fini avrà un’elevata superficie di sviluppo perciò una porosità elevata (anche superiore al 50%) e un rapporto fra micro e macropori nettamente a favore dei primi.
In seguito a tutto quanto esposto, viene dimostrato perché una muratura, benché potenzialmente porosa ed idealmente capillare, non permetterebbe di fatto di far risalire l’acqua (e quindi l’umidità) oltre limiti molto circoscritti e comunque mai oltre ai livelli teorici di pochi decimetri dal suolo.
E’ pertanto generalmente errato definire il fenomeno di risalita umida in una muratura come umidità capillare.
Centro ricerche IgroDry


